Right now, one of the most incredible and interesting developments in the world of high-tech is unfolding in the biotech sector.
Over the last couple of weeks, we saw the approval of the world’s first CRISPR genetic editing-based therapy.
This breakthrough is incredible. Yet the limited headlines published only tell a fraction of the story.
To catch up, here is the string of relevant events that occurred over the last month:
While Vertex Pharmaceuticals (VRTX) received most of the attention for the approvals and opinion, the company behind the development of Casgevy is CRISPR Therapeutics (CRSP).
Aside from the incredible improvement in health this will have on those who suffer from sickle cell disease (SCD) and transfusion dependent beta thalassemia (TDT), the real story is going untold…
The real story is about who actually owns the intellectual property and patents that underly this incredible genetic editing technology.
Because it isn’t CRISPR Therapeutics (CRSP).
For critical background on the patent battle between The Broad Institute of MIT and Harvard versus the University of California Berkeley, please refer to my Friday December 1, 2023 Ask Me Anything (AMA) issue.
I published the issue on December 5, 2023 — three days before the FDA approved Casvegy, the first CRISPR genetic editing therapy approved in the U.S in history. My AMA can be found by clicking here.
In the middle of all this excitement, the most interesting and important part of the story — and the part that still isn’t being reported — is that the technology behind this newly approved CRISPR genetic editing therapy is actually owned by another company: Editas Medicine (EDIT).
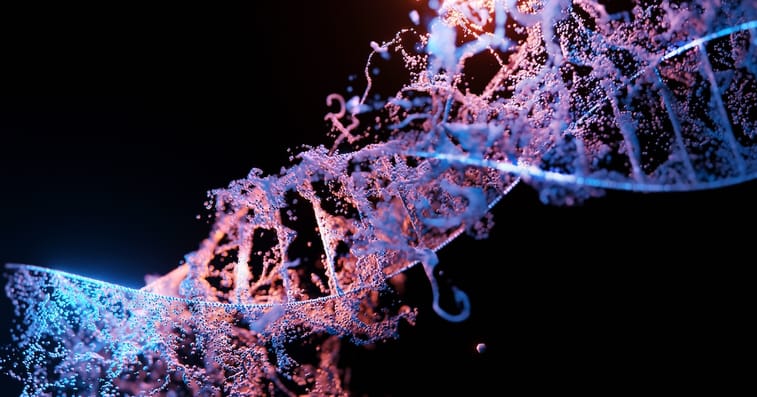
As a quick reminder, CRISPR genetic editing technology is akin to software programming for the DNA of plants, animals, and humans. It enables the “editing” of mutations in DNA that cause disease.

For technology that stretches the outer limits of what’s possible in biotech, it’s actually pretty simple when explained at a high level.
In the image above, the desired DNA sequence (in orange) is delivered to the correct place in a strand of DNA. It’s delivered via a guide RNA (in yellow). And the CRISPR therapy uses a protein (in gray) to “cut” the DNA and insert the desired DNA sequence.
I was the first analyst to begin covering CRISPR genetic editing technology, as well as Editas Medicine — a company that I had been tracking since its founding in 2013.
In December of 2014, Editas Medicine received the exclusive license of foundational CRISPR and CRISPR-Cas9 intellectual property from The Broad Institute.
This will go down as one of the smartest and most lucrative licensing agreements in history.
At that time, the implications were that if CRISPR genetic editing technology was successfully developed into therapies for human disease, the technology would have to be licensed directly from Editas.
Back then, no one knew what this was worth. Most industry experts were extremely skeptical that the tech would be safe enough to be used in humans.
I wasn’t. I knew it was worth billions, and since Editas had the exclusive rights to the foundational intellectual property, it would be worth even more.
I’ve long maintained, since 2015, that Editas would be the beneficiary of commercialization of CRISPR-based therapies.
Not only did Editas have the rights to the foundational patents, it has added new patents to its intellectual property portfolio every year since then.
And these Broad/Editas patents prevailed not once, but three times in court, from a greedy attempt by U.C. Berkeley to try and gain priority for its own patents through patent interference lawsuits and related appeals. (Again, I recapped this battle to its finality right here.)
And as recently as December 5th, days before the FDA approved Casgevy, I made the following point:
It’s the first CRISPR-based therapy to receive regulatory approval in history. Not surprisingly, CRISPR Therapeutics’ (CRSP) stock price jumped from $38.93 to $71.61 on the news. CRISPR Therapeutics is now worth about $4 billion as a result…
And what no one is writing about is: The reality is that CRISPR Therapeutics MUST license intellectual property from Editas Medicine now in order to sell its CRISPR-Cas9 based therapy.
— Jeff Brown, Outer Limits, December 5th, 2023
Which is why it came as no surprise when Editas Medicine announced last week, on December 13, 2023 — a week after my AMA — that it had entered into a non-exclusive licensing agreement with Vertex for Editas’ CRISPR-Cas9 genetic editing.
The non-exclusive agreement is for ex vivo therapies targeting the BCL11A gene for sickle cell disease (SCD) and transfusion dependent beta thalassemia (TDT).
This licensing deal received almost no coverage whatsoever.
Yet it is huge.
To recap so far…
Casgevy is the successful and now-approved CRISPR-based therapy that resulted from the partnership between CRISPR Therapeutics (CRSP) and Vertex.
Vertex got most of the headlines, as it is the larger biopharma company responsible for the filings and ultimately the marketing and distribution of the therapy.
According to the December 13th SEC filings by Editas, Vertex will pay Editas $50 million upfront. And Editas is eligible to receive an additional $50 million upfront payment (contingent on some undisclosed terms).
In addition, annual licensing fees between $10 million and $40 million will be paid to Editas through 2034.
What’s that all worth?
Somewhere in a range between $210 million and $540 million through 2034.
Licensing fees like these are almost always linked to sales associated with the therapy. Given the therapy price tag ($2.2 million per patient) and approvals in the U.K., U.S., and EU (forthcoming), I expect Editas’ payouts will be at the higher end of that range.
Editas went so far to state that the deal extends its cash runway through 2026 at a minimum.
That means that it has enough capital now to fund operations, its own therapeutic development, and continuing to increase its own intellectual property portfolio through 2026, without the need to raise any additional capital. Fantastic.
It’s a massive deal. And it is even bigger than it seems.
The rub hides within the specificity of the licensing agreement.
Editas will likely receive around a half a billion dollars in licensing fees — entirely gross margin by the way — for the use of its technology for a single gene applied towards two human diseases (SCD and TDT).
And those therapies are only for use in ex vivo approaches. Ex vivo is when the cells are taken out of the patient, edited, and then injected back into the patient.
That means that if Vertex, or CRISPR Therapeutics, wants to work with another gene, another disease, or develop an in vivo therapy, it must return to Editas and take out additional licenses.
Again, the agreement is non-exclusive, which means Editas maintains its right to license the same technology to other companies — not just limited to Vertex and CRISPR Therapeutics.
Editas is sitting on an absolute gold mine. And no one is talking about it.
And the fact that Vertex stepped up to sign this licensing deal days after receiving approval from the FDA tells us everything.
Vertex knew it had to license Editas’ patents. Otherwise it would suffer a lawsuit from Editas that it knew it would lose.
Other biopharma companies with CRISPR-based therapies in their clinical pipelines are faced with the same reality as Vertex.
U.C. Berkeley was too greedy. It wasted its time with patent infringement suits when it could have been collaborating with Editas and other leaders in the industry. Together, they could have set up a patent pool that would have benefited all players.
Editas would have still earned the largest stake in the patent pool, but at least multiple parties would have benefited, and it would have simplified licensing for CRISPR-based therapies for the entire biopharma industry.
And while I still believe a patent pool is likely, Editas has now been anointed the gatekeeper.
Meanwhile, despite these latest developments, a massive distortion still remains…
Editas Medicine trades at a valuation of just $583 million. CRISPR Therapeutics trades at $3.81 billion — 6.5-times higher.
I think we all know what that means.
What do you think of this issue of Outer Limits? As always, we welcome your feedback and questions, and look forward to them. We read each and every email. Please write to us by clicking here.